-
Ruthenium(II) Coordination Chemistry of a Fused Donor-Acceptor Ligand: Synthesis, Characterization and Photoinduced Electron Transfer Reactions of [{Ru(bpy)2}n(TTF-ppb)](PF6)2n (n = 1, 2)

C. Goze, N. Dupont, E. Beitler, C. Leiggener, H. Jia, P. Monbaron, S.-X. Liu, A. Neels, A. Hauser and S. Decurtins
Inorganic Chemistry, 47 (23) (2008), p11010-11017


DOI:10.1021/ic801252t | unige:3564 | Abstract | Article HTML | Article PDF

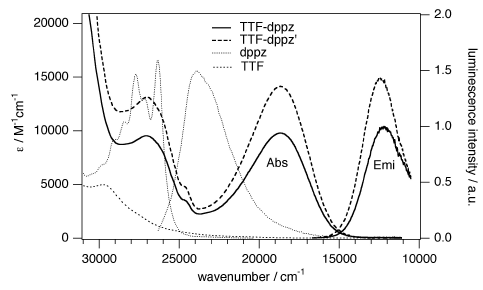
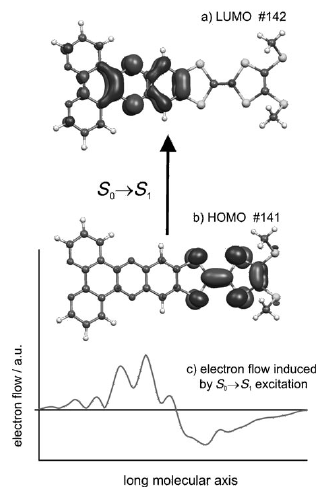
A π-extended, redox-active bridging ligand 4′,5′-bis(propylthio)tetrathiafulvenyl[i]dipyrido[2,3-a:3′,2′-c]phenazine (L) was prepared via direct Schiff-base condensation of the corresponding diamine−tetrathiafulvalene (TTF) precursor with 4,7-phenanthroline-5,6-dione. Reactions of L with [Ru(bpy)2Cl2] afforded its stable mono- and dinuclear ruthenium(II) complexes 1 and 2. They have been fully characterized, and their photophysical and electrochemical properties are reported together with those of [Ru(bpy)2(ppb)]2+ and [Ru(bpy)2(μ-ppb)Ru(bpy)2]4+ (ppb = dipyrido[2,3-a:3′,2′-c]phenazine) for comparison. In all cases, the first excited state corresponds to an intramolecular TTF → ppb charge-transfer state. Both ruthenium(II) complexes show two strong and well-separated metal-to-ligand charge-transfer (MLCT) absorption bands, whereas the 3MLCT luminescence is strongly quenched via electron transfer from the TTF subunit. Clearly, the transient absorption spectra illustrate the role of the TTF fragment as an electron donor, which induces a triplet intraligand charge-transfer state (3ILCT) with lifetimes of approximately 200 and 50 ns for mono- and dinuclear ruthenium(II) complexes, respectively.